Abstract
Background: Inhibitor formation is a serious complication of hemophilia A, occurring in up to 30% with severe disease. It is associated with a T-cell response to infused factor VIII which neutralizes and renders it ineffective. Bypass therapy (rVIIa, FEIBA) is less effective, resulting in high morbidity and mortality. While immune tolerance (ITI) with high-dose FVIII neutralizes the inhibitor, it is costly, invasive, and unsuccessful in 30%. Basic studies suggest rFVIIIFc, Eloctate, is less immunogenic than standard recombinant FVIII, rFVIII, as the Fc portion contains regulatory T cells that promote tolerance. In the hemophilia mouse model, rFVIIIFc reduces inhibitor frequency, lowers titer, and shortens ITI, but little is known of its impact on inhibitor patients, as they were excluded from clinical trials.
Methods: We, therefore, reviewed outpatient medical records on 60 patients with hemophilia A with and without inhibitors, cared for at the Hemophilia Center of Western Pennsylvania between January 1, 2006 and June 1, 2017, who were treated with rFVIIIFc and on whom anti-FVIII inhibitor assay results were available. This study was approved by the University of Pittsburgh IRB as an expedited study, PRO17020097. Means, medians, and standard deviations or frequencies (percentages) were determined for clinical variables, including age, race, proportion developing inhibitors, peak titer, proportion achieving tolerance, and time to tolerance on ITI. Comparisons between patients with high-titer, low-titer, and no inhibitors were by student's t test for continuous data, or chi-square or Fisher's exact test for discrete data. A p-value of < 0.05 was considered statistically significant.
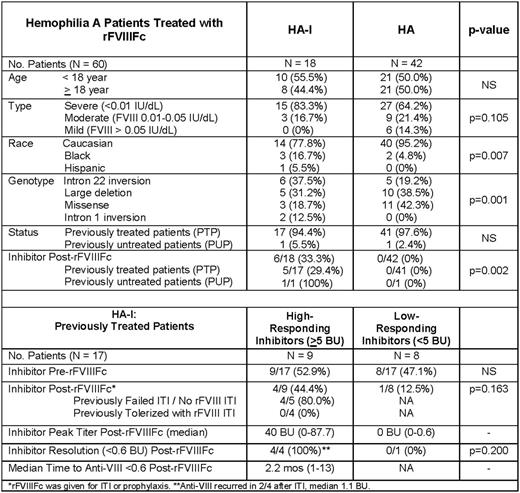
Results: The 60 patients whose charts were reviewed for this study included 18 (30.0%) with hemophilia A with an inhibitor (HA-I), of whom 9 had high-responding inhibitors (≥5 BU) and 9 had low-responding inhibitors (<5 BU); and 42 (70.0%) with hemophilia A without an inhibitor (HA). HA-I were similar to HA in age, with 44.4% vs. 50.0% ≥ 18 years of age, respectively, p>0.5, and in disease severity, 83.3% vs. 64.2% < 0.01 IU/dL, p=0.105, but a greater proportion were black, 16.7% vs. 4.8%, p=0.007, and had an intron 22 inversion mutation, 37.5% and 19.2%, p=0.001 (Table). The majority of both groups were previously treated patients (PTPs), 17 of 18 HA-I (94.4%) vs. 41 of 42 HA (97.6%). Following initiation of rFVIIIFc, none of 42 HA, 0/42 (0%) developed inhibitors, while, by contrast, inhibitors were detected in 6 of 18 (33.3%) HA-I, p=0.002. The 6 patients developing inhibitors after rFVIIIFc included 5 PTPs and 1 PUP: the single PUP was from an inhibitor-prone kindred with an exon 14 nonsense variant (75% inhibitor risk) (Haemophilia, 2016), and developed a low-responding inhibitor, 1.4 BU, after 10 exposures to rFVIIIFc, which resolved to <0.6 BU within 1 month while continuing rFVIIIFc. The 5 PTPs developing inhibitors on rFVIIIFc included 4 of 9 (44.4%) previous high-responding inhibitors (≥5 BU) and 1 of 8 (12.5%) previous low-responding inhibitors (<5 BU). All 4 past high-responders had previously failed or had never undergone immune tolerance induction (ITI); while the single past low-responder had longstanding fluctuating low-level inhibitor titers. Following initiation of rFVIIIFc, the 4 PTPs with past high-responding inhibitors developed inhibitors, median anti-FVIII = 40 BU (0-87.7), which resolved, in all 4, to anti-VIII <0.6 BU after a median 2.2 months (1-13) while continuing rFVIIIFc; the 1 PTP with a past low-responding inhibitor and chronic fluctuating low-level inhibitor, developed a low-responding inhibitor 0.6 BU (0-0.6 BU), which resolved to <0.6 BU while continuing rFVIIIFc, but continues to fluctuate between 0 and 0.6 BU.
Discussion: These findings indicate that PTPs without inhibitors or with tolerized inhibitors do not develop inhibitors after switching to rFVIIIFc. By contrast, most PTPs with high-responding inhibitors who failed or never underwent ITI, develop inhibitor recurrence after switching to rFVIIIFc, but these are rapidly tolerized while continuing rFVIIIFc. In PTPs with low-responding inhibitors, rFVIIIFc may reduce but does not eliminate fluctuating low-level titers. Finally, in PUPs at risk for inhibitor formation, rFVIIIFc may induce low-responding low-titer inhibitors which are rapidly tolerized while continuing rFVIIIFc.
Ragni: SPARK: Research Funding; NovoNordisk: Honoraria; Alnylam: Consultancy, Honoraria, Research Funding; Sangamo: Research Funding; Genentech/Roche: Research Funding; Bioverativ: Consultancy, Honoraria, Research Funding; MOGAM: Consultancy, Honoraria; Bayer: Consultancy, Honoraria, Research Funding; Biomarin: Consultancy, Honoraria, Research Funding; Shire: Consultancy, Honoraria, Research Funding. Malec: Bioverativ: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal